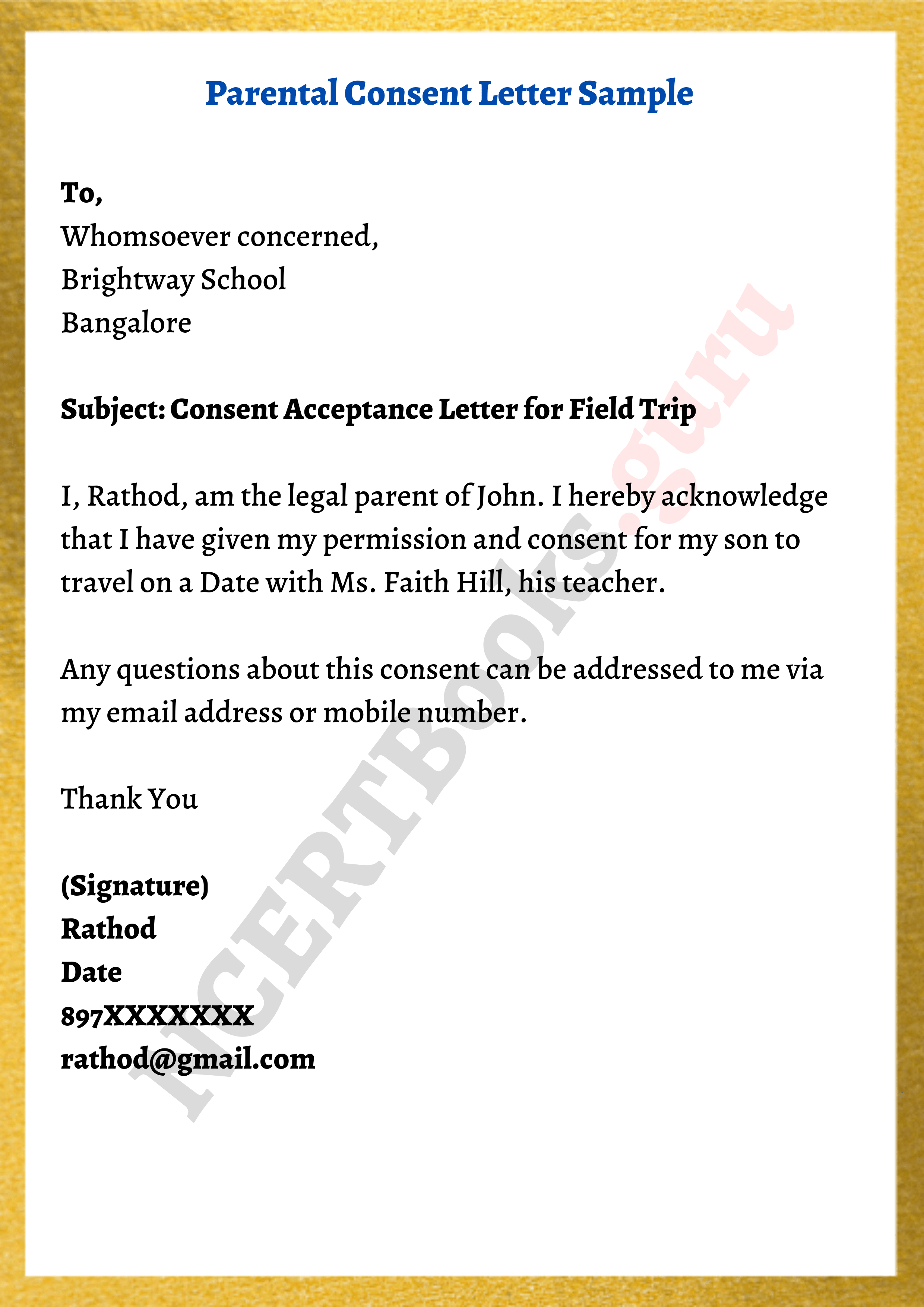
Formidable Tips About How To Write A Consent Form

However, first person or ‘i’ should be used on the last page in the “consent to participate” section,.
How to write a consent form. Using large language models to write documents for clinical trials. Begin by introducing yourself and the purpose of the. The legal side of the business is often difficult to navigate, so at least you can save some time with the technical setup of getting.
How to make a consent form. Kick off your consent form by outlining your study's purpose and objectives. It says that ai systems that can be used in different applications are.
A study description and purpose. Sample consent and permission forms. Now that we have a clear understanding of the purpose and components of a consent form, let`s explore some tips for writing one that.
For example, clicking “i agree” on a website or. A consent form is a document that explains the procedures, risks, and benefits of a research project to the participants. Follow these steps to write an effective consent form.
How do you write a consent form for user. The informed consent form must be written in language easily understood by the subjects, it must minimise the possibility of coercion or undue influence, and the. To ensure consent is valid, you need to ensure that it is “freely given, specific, informed and unambiguous”, signified by a “clear, affirmative action” (art.
From rags to riches: Find the required elements, tips, and templates for different types of research and populations. Updated on june 09, 2022.
It should be clear, concise, and in the 2nd person. Recruitment documents help people make informed choices about whether to participate in a research study. Here’s a basic format for informed consent that can be customized for specific research studies:
The consent form should be written in the second person. A consent form template for ux research studies what is an informed consent form? Writing a participant information sheet and consent form.
And iv) the ongoing or continuing nature of permission. If you prefer, consent forms can be written in the language which the researcher is most comfortable with and translated into the participants' mother tongue and english. Briefly explain why the study’s being conducted.
Let’s use a dental consent form as our example document here. Every group or organisation in the uk has a duty to safeguard children and young people in. Example consent form for activities and events.
















![FREE Consent Form [PDF, WORD]](https://images.sampleforms.com/editor/wp-content/uploads/2020/07/Consent-Form.png)
