Fabulous Tips About How To Easily Balance Chemical Equations
A balanced equation shows the ratio in which the reactants react, and the ratio in which the products are produced.
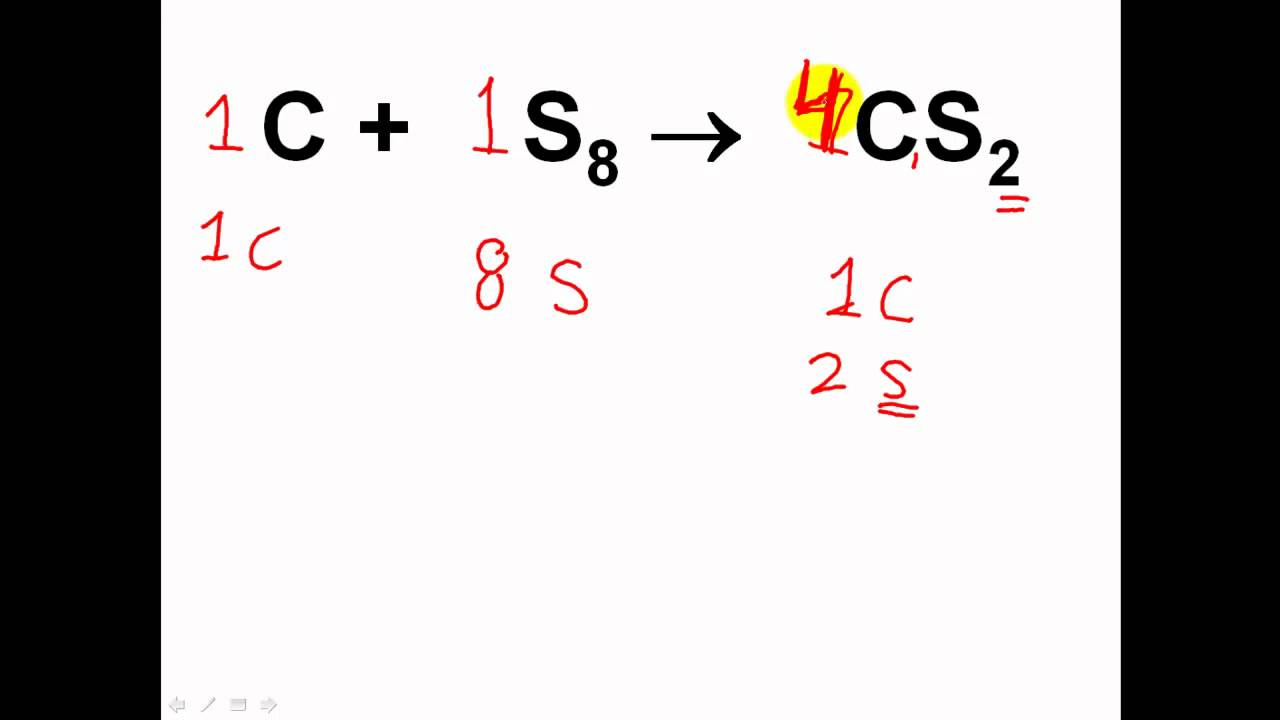
How to easily balance chemical equations. So, the product and reactants of chemical equations should have an equal number of atoms on both sides. In this example, we balance the combustion reaction of ethylene, c₂h₄, and provide tips on how to balance more complex chemical equations. Write down number of atoms.
The first method of balancing chemical equations is taught in school. Steps of balancing a chemical equation identify each element found in the equation. Fe, au, co, br, c, o, n, f.
Use uppercase for the first character in the element and lowercase for the second character. Identify the products and reactants the first step in balancing a chemical equation is to identify your reactants and your products. The unbalanced equation must be obtained from the chemical formulae of the reactants and the products (if it is.
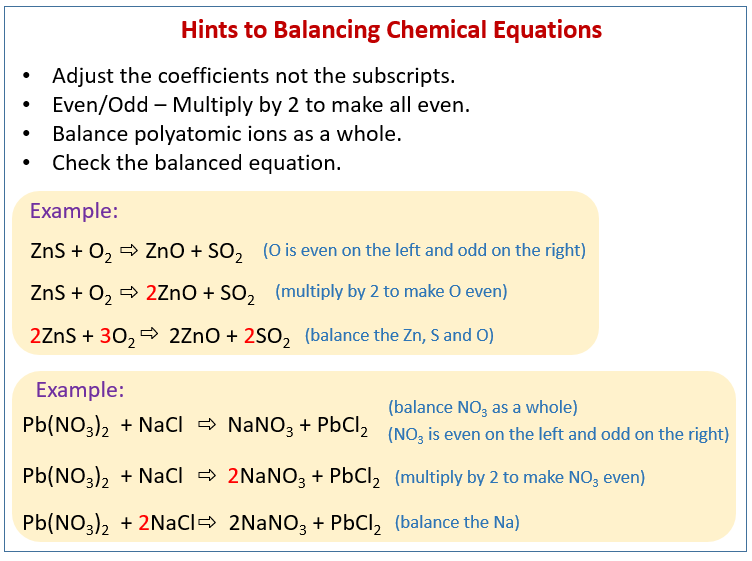
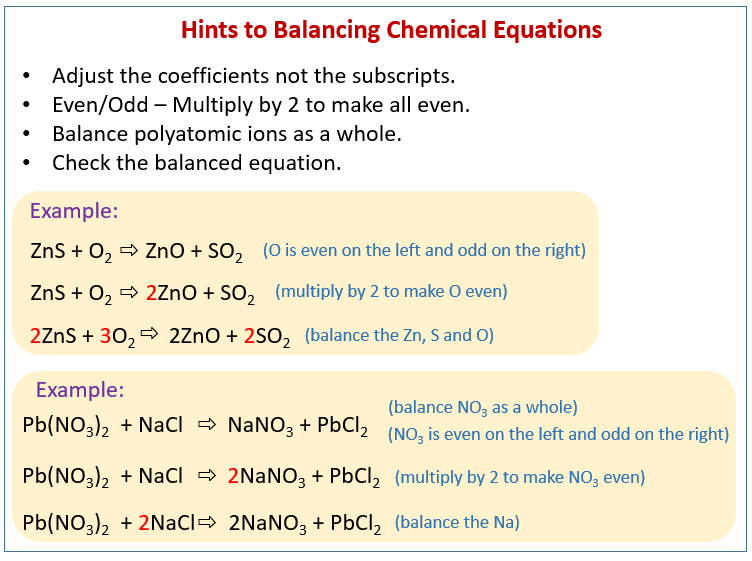
The balanced equation will appear above. The simplest and most generally useful method for balancing chemical equations is called “balancing by inspection,” also known as trial and error. This method of balancing chemical equations is simple and easy to understand.
The products are on the right side. Ionic charges are not yet supported and will be ignored. Examples of complete chemical equations to balance:
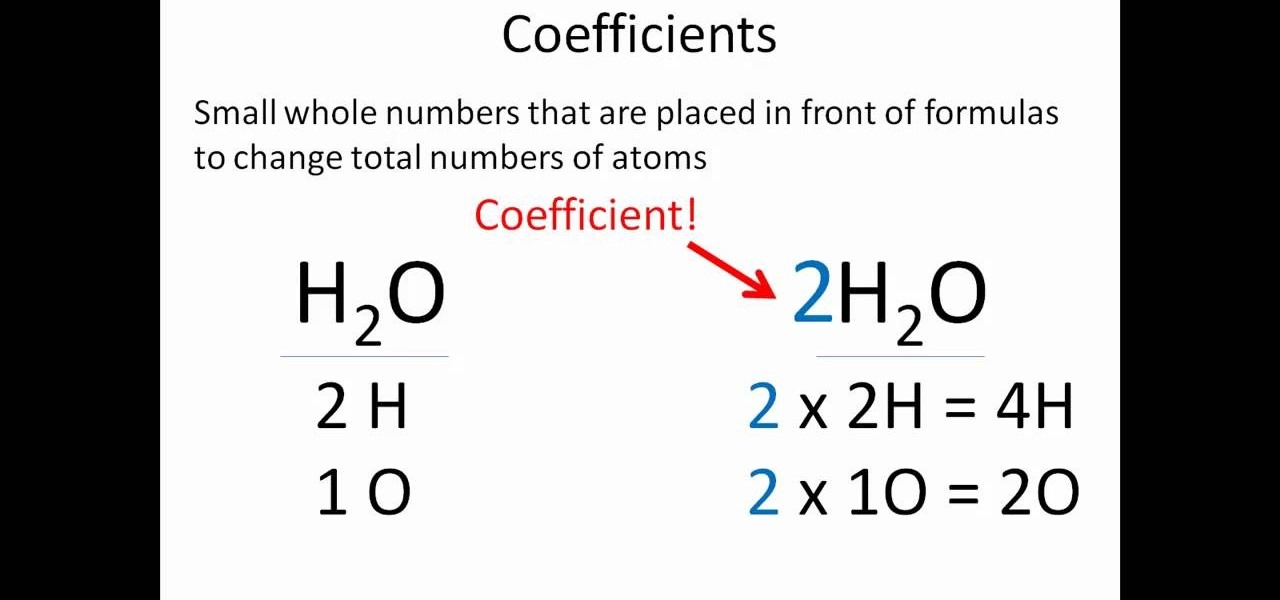
Methods of balancing chemical equations. Then start solving the system of equations to get the following values: If an equation is balanced and if a chemical doesn’t have a coefficient, then we assume it has a coefficient of 1.
Balancing chemical equations in five easy steps balancing chemical equations is a core skill in chemistry. Being able to balance chemical equations is a vital skill for chemistry. For example, your equation should look something like h2 + o2 → h2o.
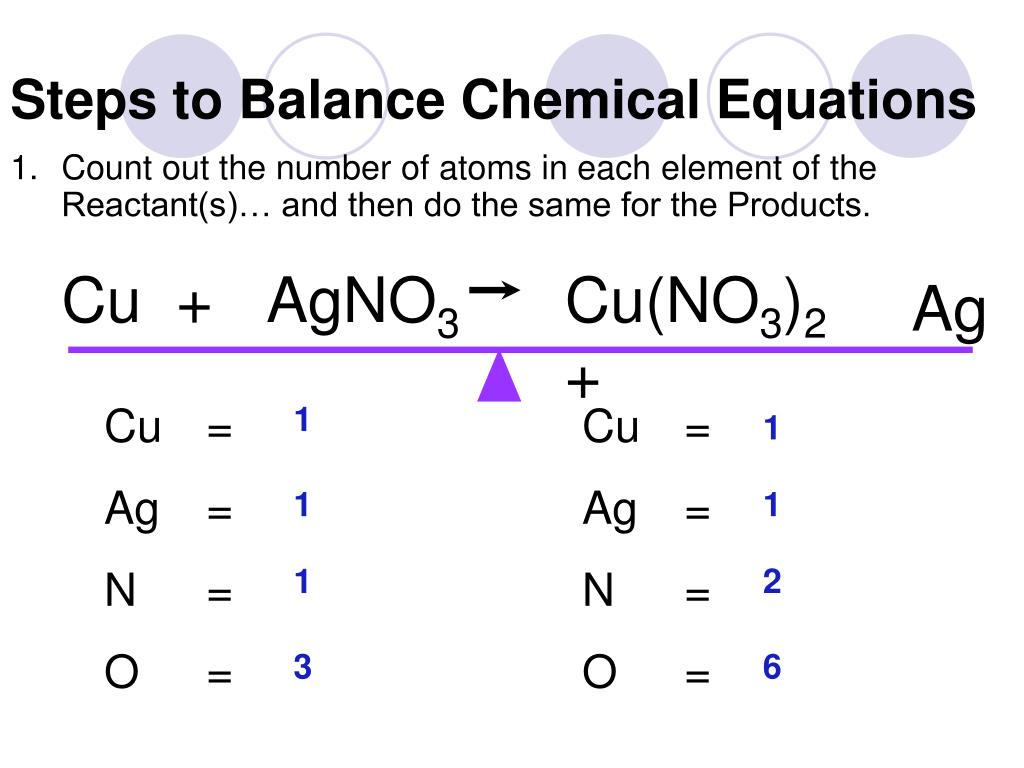
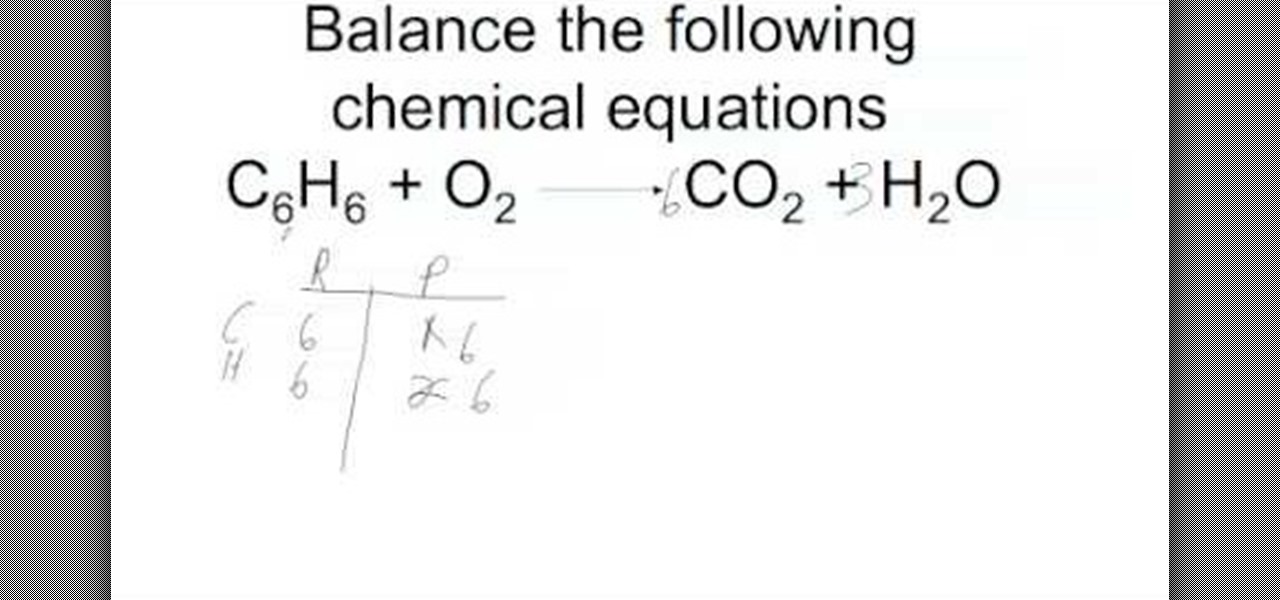
Write the unbalanced chemical equation. The ultimate goal for balancing chemical equations is to make both sides of the reaction, the reactants and the products, equal in the number of atoms per element. Here's a look at the steps involved in balancing equations, plus a worked example of how to balance an equation.
2b = 3c + d, we can calculate b like this: The trial and error or atom counts method makes the procedure quite lengthy. As per the law of conservation of mass, “atom can neither be created nor can be destroyed”.
Fe + cl 2 = fecl 3 kmno 4 + hcl = kcl + mncl 2 + h 2 o + cl 2 k 4 fe (cn) 6 + h 2 so 4 + h 2 o = k 2 so 4 + feso 4 + (nh 4) 2 so 4 + co c 6 h 5 cooh + o 2 = co 2 + h 2 o k 4 fe (cn) 6 + kmno 4 + h 2 so 4 = khso 4 + fe 2 (so 4) 3 + mnso 4 + hno 3 + co 2 + h 2 o Remember, your reactants are on the left side of your equation. Write the unbalanced equation to show the reactants and products.








:max_bytes(150000):strip_icc()/BalanceEquations2-56a132765f9b58b7d0bcf538.png)