Best Info About How To Tell If A Reaction Is Redox

If the reaction does occur, write the products of the reaction and balance.
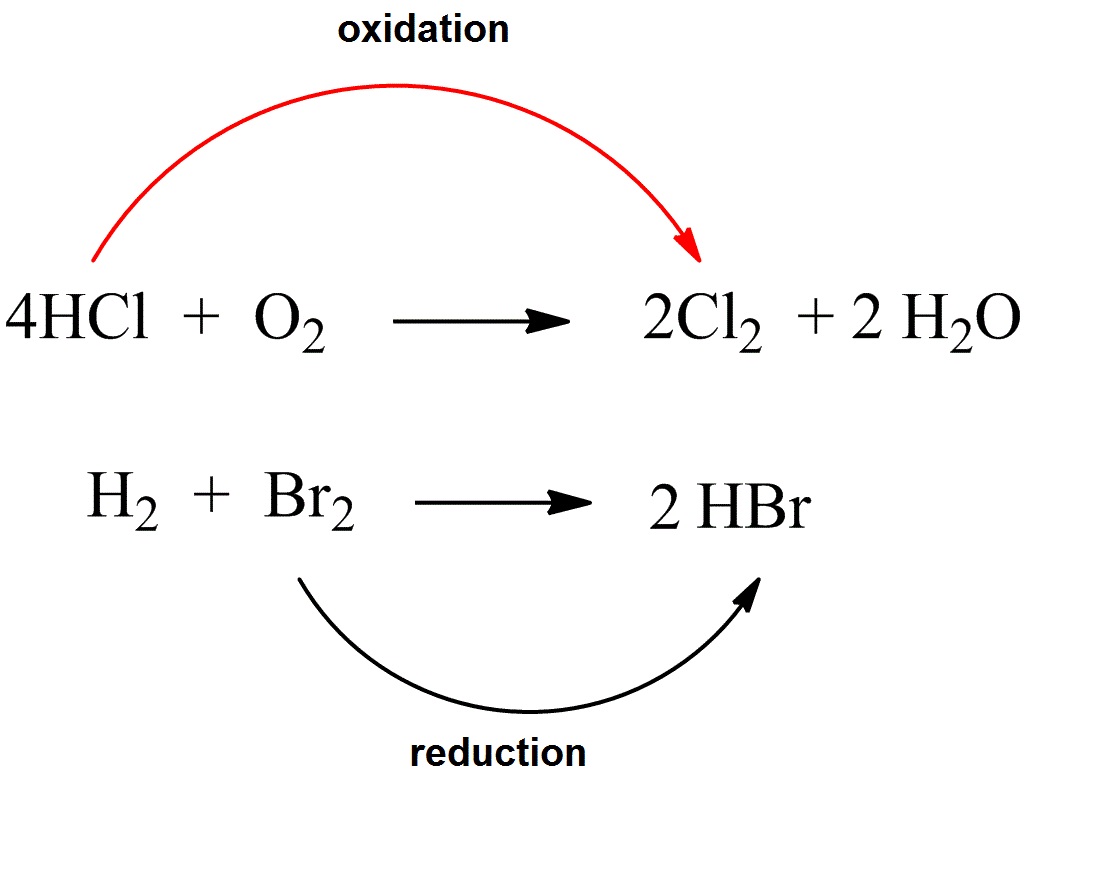
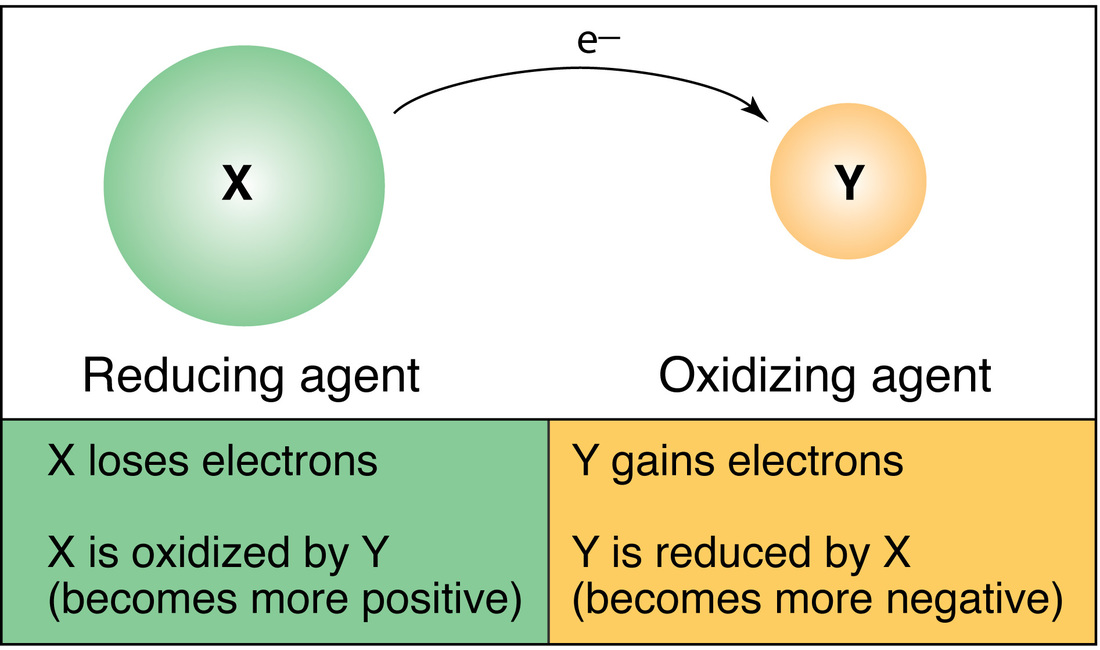
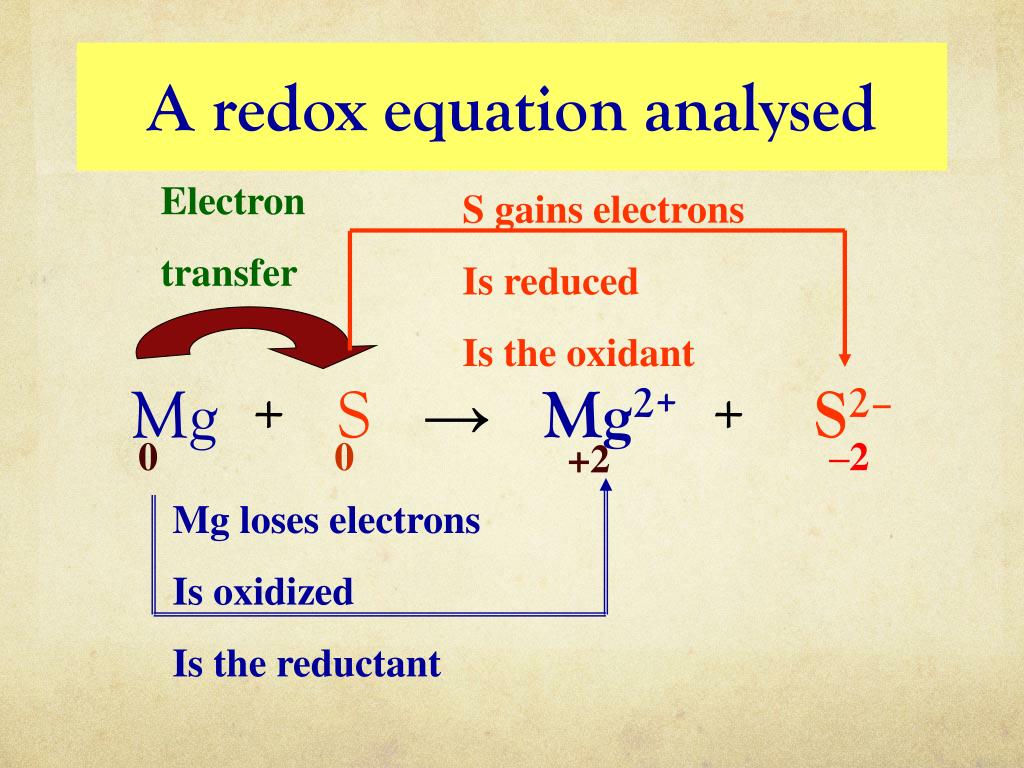
How to tell if a reaction is redox. How to identify a redox reaction? The total number of electrons being lost by sodium (two, one lost from each na atom) is gained by bromine. 5k views 3 years ago the mole and equations.
This video is about redox and nonredox reactions. In summary, redox reactions can always be recognized by a change in oxidation number of two of the atoms in the reaction. Identifying oxidation and reduction.
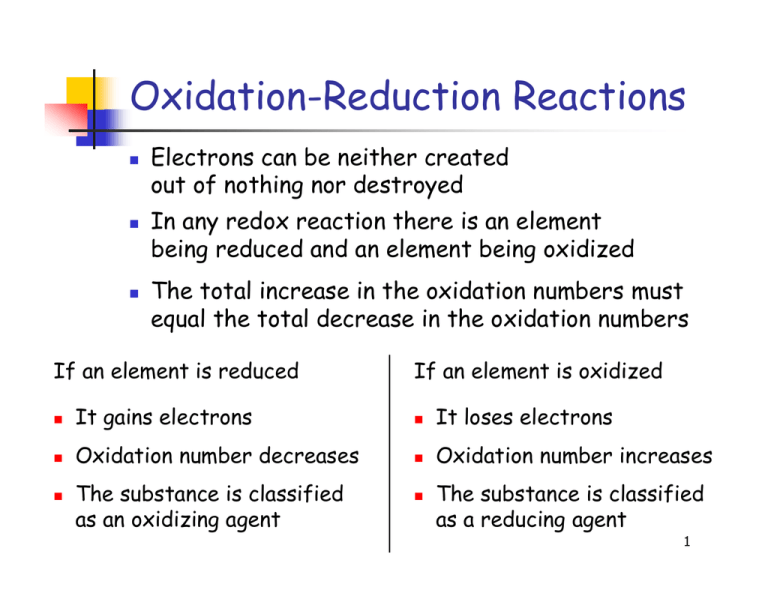
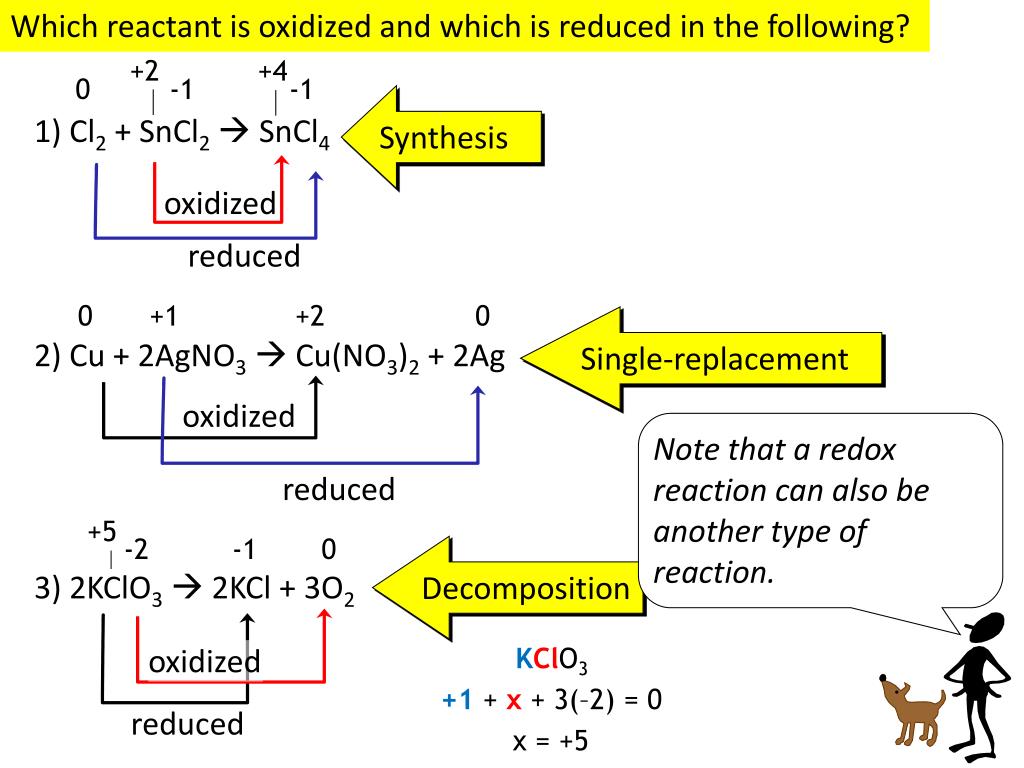
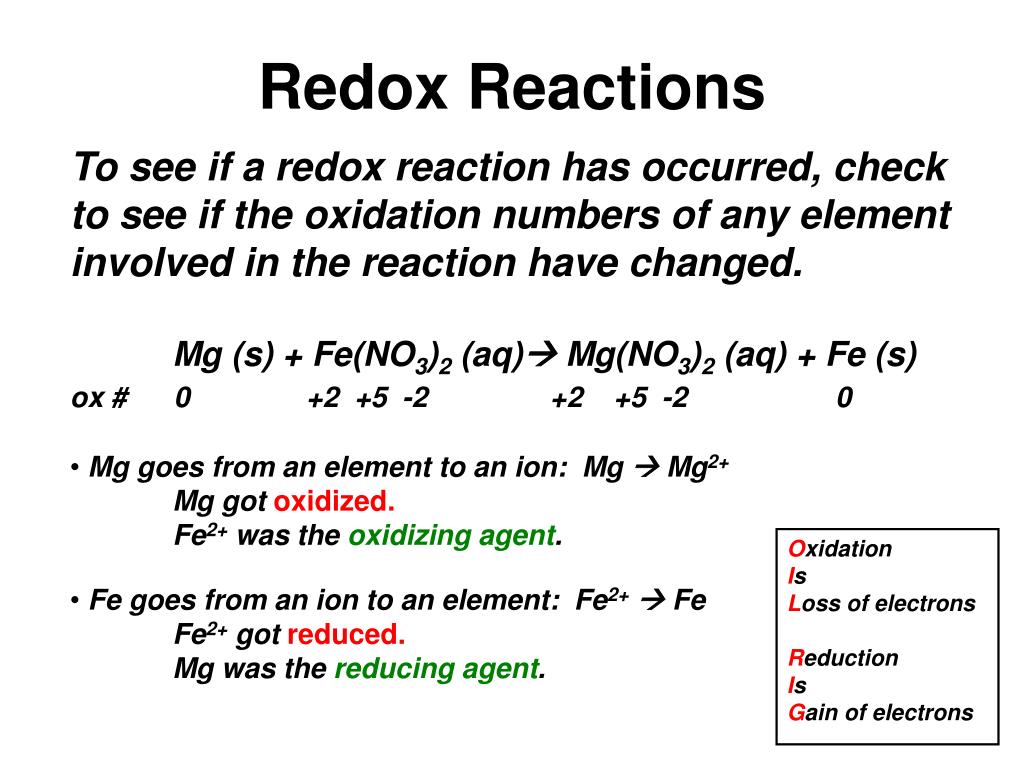
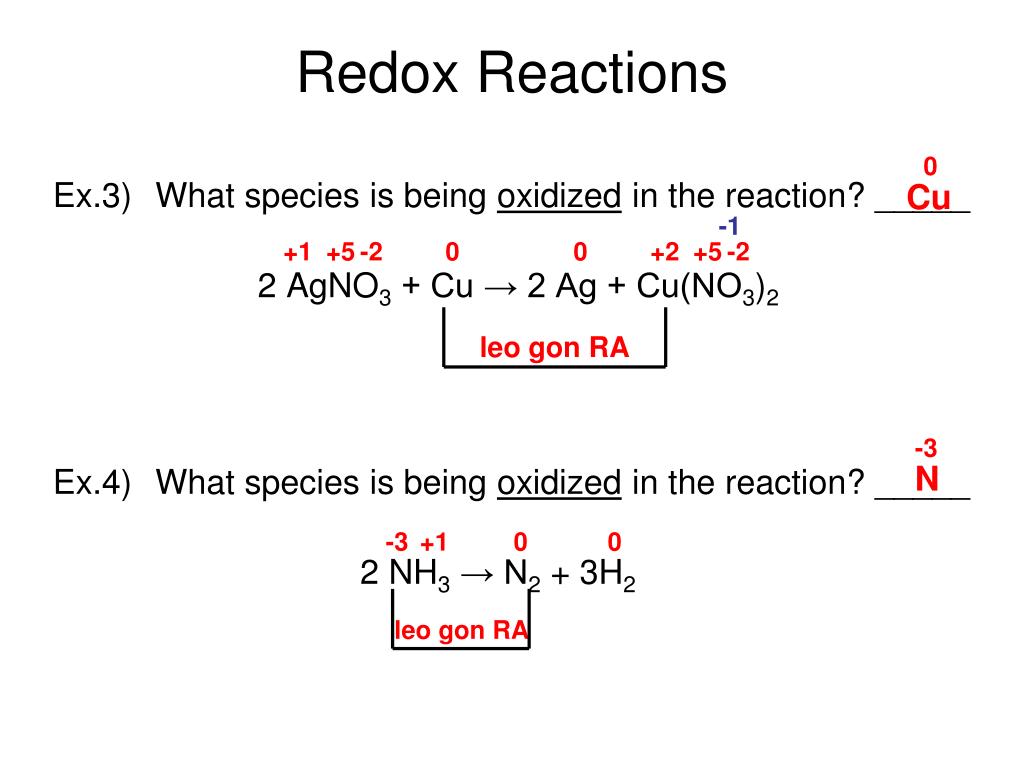
Identify which species is oxidized and which is reduced by looking at the changes in oxidation numbers. One way is to look at the change in free energy (g) for the reaction. Oxidation and reduction.
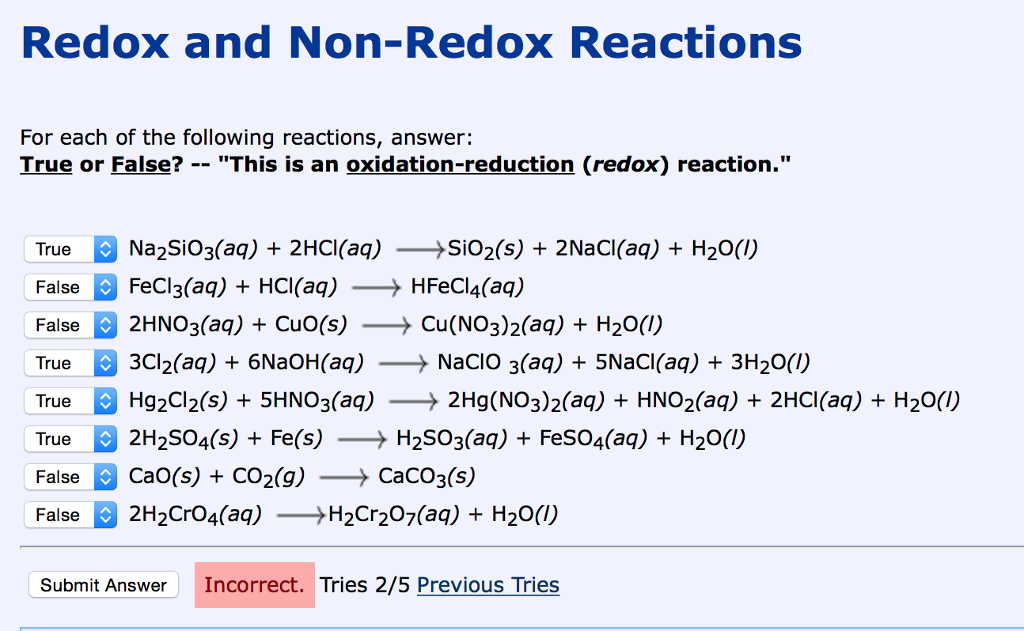
How to tell if redox. Use the activity series to predict if the following reactions will occur. To identify a redox reaction, we must first calculate the oxidation number of each atom in the reaction.
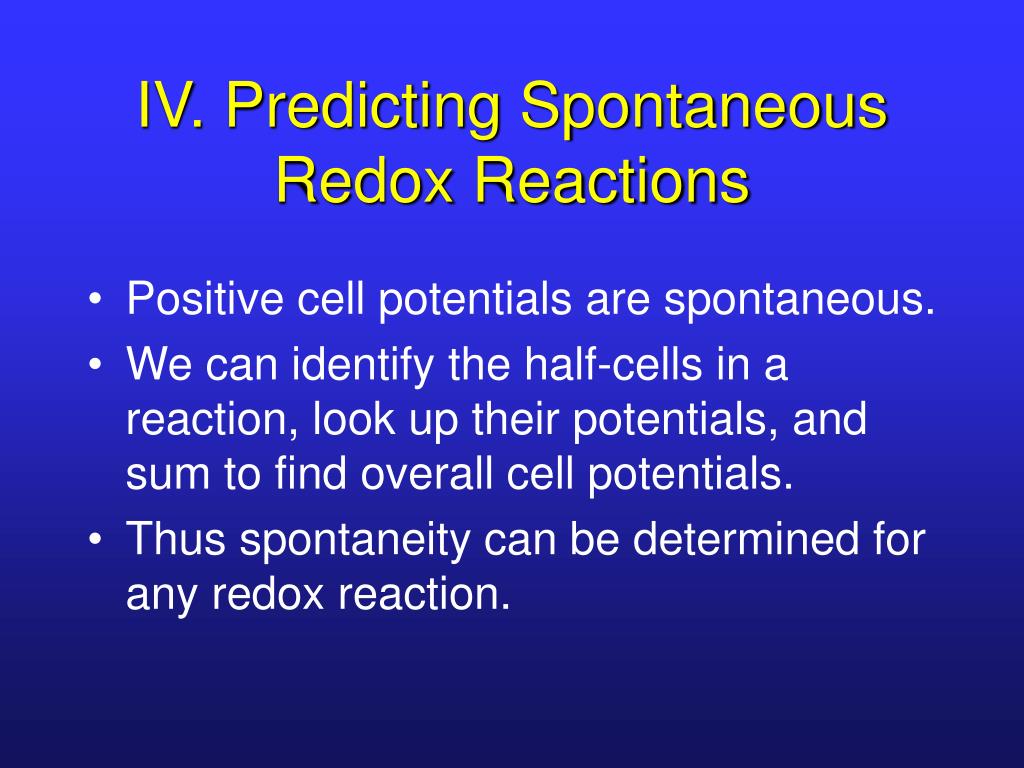
There are a few ways to tell if a redox reaction is spontaneous. A redox equation can be balanced using the following stepwise procedure: If there is no change in oxidation number, then the reaction is not a.
Before we look at redox reactions we need to first learn how to tell if a reaction is a redox reaction. Oxidation involves an increase in oxidation number, while reduction involves a decrease in oxidation number. If g is negative, the reaction is.
If any of the oxidation numbe. Modified 5 years, 7 months ago. This page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions.
How can you determine if a given chemical reaction is a redox reaction or not? How to tell if a reaction is a redox reaction. In grade 10 you learnt that a redox reaction involves a change in the charge.
For each of the reactions given below, calculate the oxidation number of each of the elements in the reactants and the products and determine if the reaction involves. Because oxidation numbers are changing, this is a redox reaction. We look at the 7 rules for assigning oxidation.
Any reaction in which no. How to identify a redox reaction?